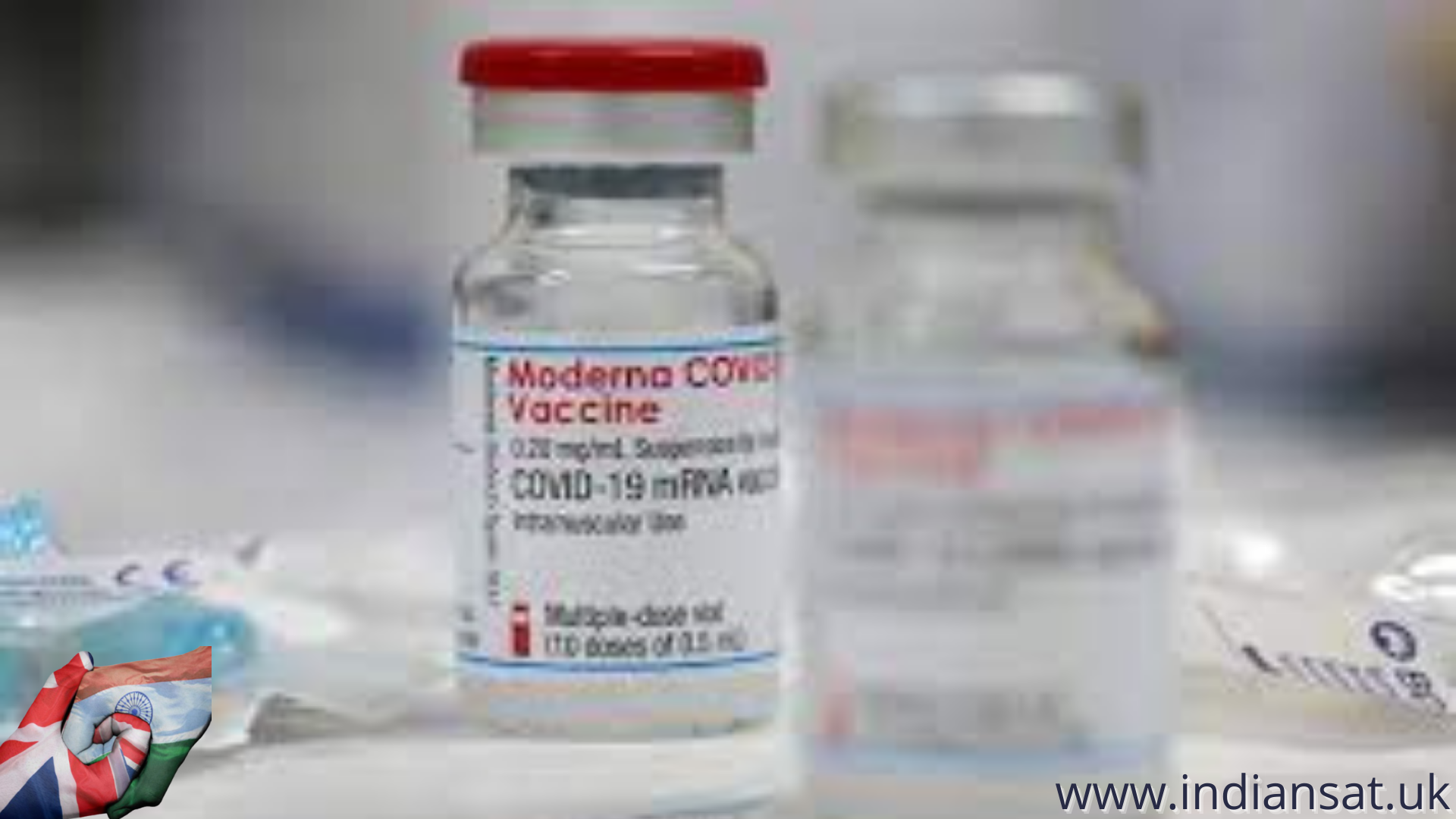
Moderna’s Covid-19 messenger RNA (mRNA) vaccine is likely to reach India this week, reported news agency ANI on Monday citing sources.
Health officials expect Moderna vaccines to reach hospitals by July 15 for administration, as per reports.
Recently, the Drugs Controller General of India (DCGI) granted permission to Mumbai-based pharmaceutical company Cipla to import Moderna’s Covid-19 vaccine for restricted emergency use in the country. “This permission is for restricted use in emergency situations in the public interest. The firm has to submit 7 days’ safety assessment of the vaccine in the first 100 beneficiaries before rolling out of the vaccine for further immunisation programme, according to the approval order.”
Moderna’s vaccine is the fourth COVID-19 jab to be available in India after Covishield, Covaxin and Sputnik.
Moderna’s two-dose vaccine has been widely used in several regions, including the United States and Europe, but may face some cost and distribution hurdles in India as the shot requires cold storage.
Moderna, through a separate communication, had informed that the US Government has agreed to donate a certain number of doses of the Moderna COVID-19 Vaccine, mRNA-1273, through COVAX to the Government of India for use in India and has submitted the dossiers through e-mail.
In a bid to expedite the rollout of vaccines, the DCGI on June 1 decided to waive off testing of batches at CDL for foreign-manufactured vaccines that have been approved by international drug regulators such as the US FDA, the UK’s MHRA or the WHO.
The Centre had in April issued detailed guidelines and proactively eased entry of foreign made COVID-19 vaccines approved by the US FDA, EMA, UK’s MHRA and Japan’s PMDA, and WHO’s Emergency Use Listing into India.
According to the guidelines, these vaccines will not need to undergo prior bridging trials. The provision was further amended to waive off the trial requirement altogether for the well-established vaccines manufactured in other countries.
![]()